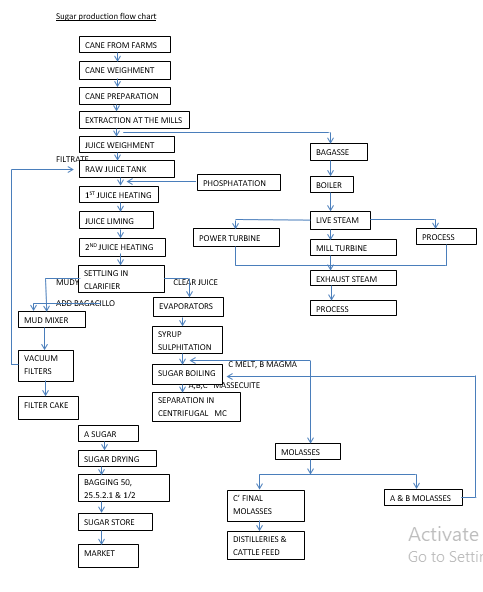
Fuel Values of Foods and Other Substances
The food we eat is broken down, or metabolized, in stages by a group of complex biological molecules called enzymes. Most of the energy released at each stage is captured for function and growth. One interesting aspect of metabolism is that the overall change in energy is the same as it is in combustion. For example, the total enthalpy change for the conversion of glucose (C 6 H 12 O 6 ) to carbon dioxide and water is the same whether we burn the substance in air or digest it in our bodies: C 6 H 12 O 6 ( s ) + 6 O 2 ( g ) → 6CO 2 ( g ) + 6 H 2 O ( l ) ΔH = - 2801 kJ/mol The important difference between metabolism and combustion is that the latter is ...